Arsenic »
PDB 1z6b-2im2 »
2e11 »
Arsenic in PDB 2e11: The Crystal Structure of XC1258 From Xanthomonas Campestris: A Cn- Hydrolase Superfamily Protein with An Arsenic Adduct in the Active Site
Protein crystallography data
The structure of The Crystal Structure of XC1258 From Xanthomonas Campestris: A Cn- Hydrolase Superfamily Protein with An Arsenic Adduct in the Active Site, PDB code: 2e11
was solved by
K.-H.Chin,
Y.-D.Tsai,
N.-L.Chan,
K.-F.Huang,
A.H.-J.Wang,
S.-H.Chou,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 104.83 / 1.73 |
| Space group | P 21 21 2 |
| Cell size a, b, c (Å), α, β, γ (°) | 143.280, 154.305, 51.158, 90.00, 90.00, 90.00 |
| R / Rfree (%) | 14 / 21.7 |
Arsenic Binding Sites:
The binding sites of Arsenic atom in the The Crystal Structure of XC1258 From Xanthomonas Campestris: A Cn- Hydrolase Superfamily Protein with An Arsenic Adduct in the Active Site
(pdb code 2e11). This binding sites where shown within
5.0 Angstroms radius around Arsenic atom.
In total 4 binding sites of Arsenic where determined in the The Crystal Structure of XC1258 From Xanthomonas Campestris: A Cn- Hydrolase Superfamily Protein with An Arsenic Adduct in the Active Site, PDB code: 2e11:
Jump to Arsenic binding site number: 1; 2; 3; 4;
In total 4 binding sites of Arsenic where determined in the The Crystal Structure of XC1258 From Xanthomonas Campestris: A Cn- Hydrolase Superfamily Protein with An Arsenic Adduct in the Active Site, PDB code: 2e11:
Jump to Arsenic binding site number: 1; 2; 3; 4;
Arsenic binding site 1 out of 4 in 2e11
Go back to
Arsenic binding site 1 out
of 4 in the The Crystal Structure of XC1258 From Xanthomonas Campestris: A Cn- Hydrolase Superfamily Protein with An Arsenic Adduct in the Active Site

Mono view
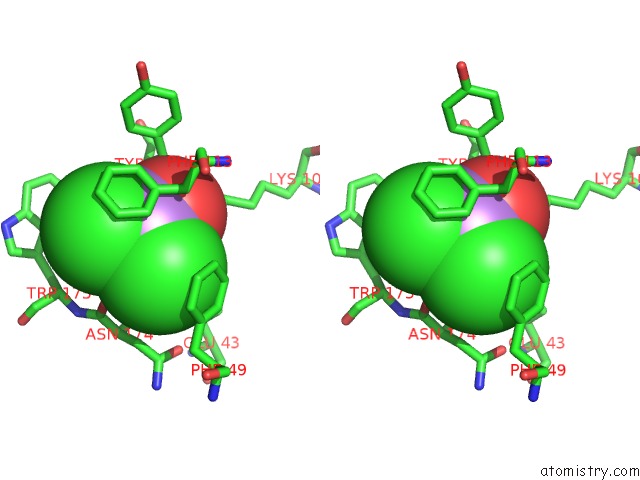
Stereo pair view

Mono view

Stereo pair view
A full contact list of Arsenic with other atoms in the As binding
site number 1 of The Crystal Structure of XC1258 From Xanthomonas Campestris: A Cn- Hydrolase Superfamily Protein with An Arsenic Adduct in the Active Site within 5.0Å range:
|
Arsenic binding site 2 out of 4 in 2e11
Go back to
Arsenic binding site 2 out
of 4 in the The Crystal Structure of XC1258 From Xanthomonas Campestris: A Cn- Hydrolase Superfamily Protein with An Arsenic Adduct in the Active Site
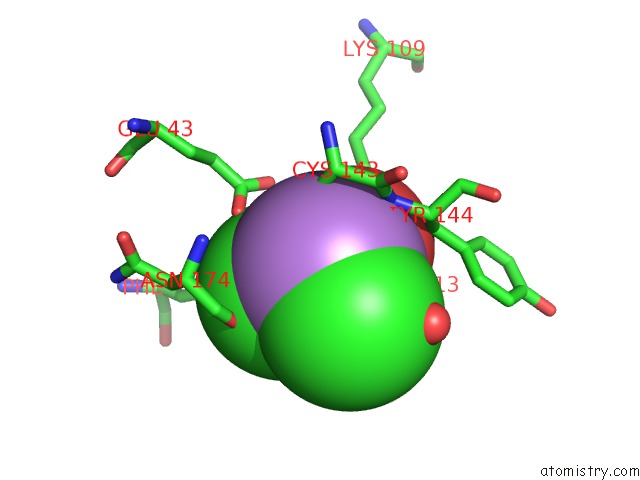
Mono view
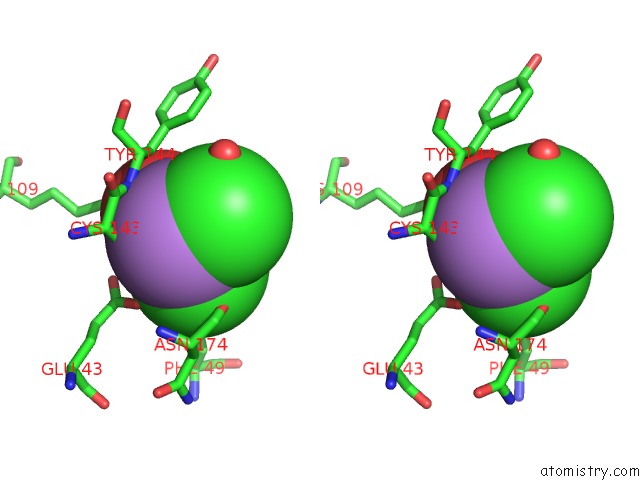
Stereo pair view

Mono view

Stereo pair view
A full contact list of Arsenic with other atoms in the As binding
site number 2 of The Crystal Structure of XC1258 From Xanthomonas Campestris: A Cn- Hydrolase Superfamily Protein with An Arsenic Adduct in the Active Site within 5.0Å range:
|
Arsenic binding site 3 out of 4 in 2e11
Go back to
Arsenic binding site 3 out
of 4 in the The Crystal Structure of XC1258 From Xanthomonas Campestris: A Cn- Hydrolase Superfamily Protein with An Arsenic Adduct in the Active Site
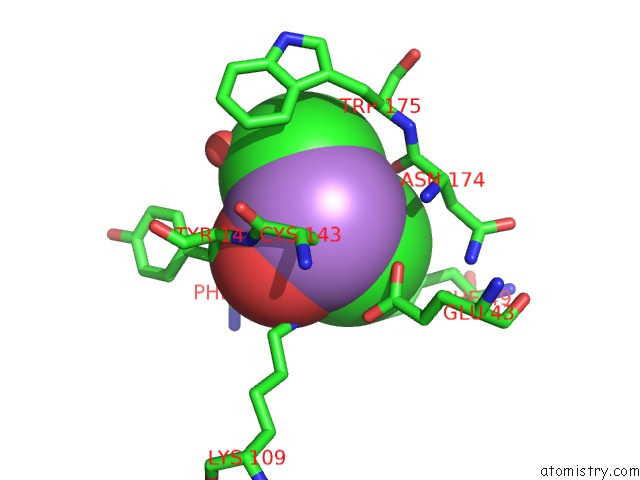
Mono view

Stereo pair view

Mono view

Stereo pair view
A full contact list of Arsenic with other atoms in the As binding
site number 3 of The Crystal Structure of XC1258 From Xanthomonas Campestris: A Cn- Hydrolase Superfamily Protein with An Arsenic Adduct in the Active Site within 5.0Å range:
|
Arsenic binding site 4 out of 4 in 2e11
Go back to
Arsenic binding site 4 out
of 4 in the The Crystal Structure of XC1258 From Xanthomonas Campestris: A Cn- Hydrolase Superfamily Protein with An Arsenic Adduct in the Active Site
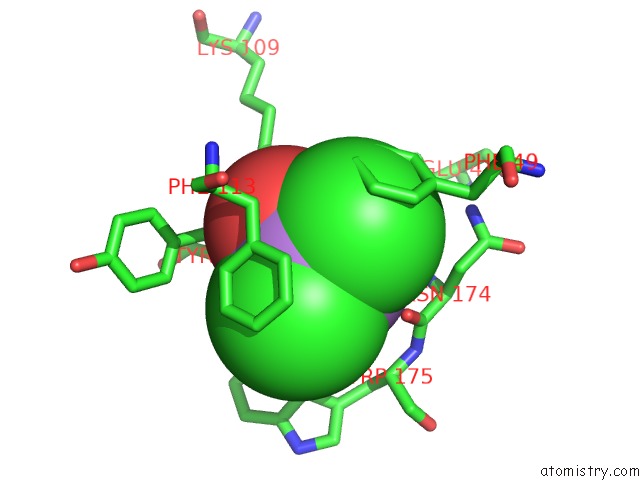
Mono view

Stereo pair view

Mono view

Stereo pair view
A full contact list of Arsenic with other atoms in the As binding
site number 4 of The Crystal Structure of XC1258 From Xanthomonas Campestris: A Cn- Hydrolase Superfamily Protein with An Arsenic Adduct in the Active Site within 5.0Å range:
|
Reference:
K.-H.Chin,
Y.-D.Tsai,
N.-L.Chan,
K.-F.Huang,
A.H.-J.Wang,
S.-H.Chou.
The Crystal Structure of XC1258 From Xanthomonas Campestris: A Putative Procaryotic Nit Protein with An Arsenic Adduct in the Active Site Proteins V. 69 665 2007.
ISSN: ISSN 0887-3585
PubMed: 17640068
DOI: 10.1002/PROT.21501
Page generated: Sun Jul 6 23:07:31 2025
ISSN: ISSN 0887-3585
PubMed: 17640068
DOI: 10.1002/PROT.21501
Last articles
F in 7LI5F in 7LHZ
F in 7LH7
F in 7LDE
F in 7LEP
F in 7LDD
F in 7LGX
F in 7LGK
F in 7LG8
F in 7LD3