Arsenic »
PDB 3g3s-3n5t »
3gwt »
Arsenic in PDB 3gwt: Catalytic Domain of Human Phosphodiesterase 4B2B in Complex with A Quinoline Inhibitor
Enzymatic activity of Catalytic Domain of Human Phosphodiesterase 4B2B in Complex with A Quinoline Inhibitor
All present enzymatic activity of Catalytic Domain of Human Phosphodiesterase 4B2B in Complex with A Quinoline Inhibitor:
3.1.4.17;
3.1.4.17;
Protein crystallography data
The structure of Catalytic Domain of Human Phosphodiesterase 4B2B in Complex with A Quinoline Inhibitor, PDB code: 3gwt
was solved by
D.O.Somers,
M.Neu,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 20.00 / 1.75 |
| Space group | I 21 21 21 |
| Cell size a, b, c (Å), α, β, γ (°) | 88.706, 94.783, 105.995, 90.00, 90.00, 90.00 |
| R / Rfree (%) | 20.5 / 25 |
Other elements in 3gwt:
The structure of Catalytic Domain of Human Phosphodiesterase 4B2B in Complex with A Quinoline Inhibitor also contains other interesting chemical elements:
| Magnesium | (Mg) | 1 atom |
| Zinc | (Zn) | 1 atom |
Arsenic Binding Sites:
The binding sites of Arsenic atom in the Catalytic Domain of Human Phosphodiesterase 4B2B in Complex with A Quinoline Inhibitor
(pdb code 3gwt). This binding sites where shown within
5.0 Angstroms radius around Arsenic atom.
In total 5 binding sites of Arsenic where determined in the Catalytic Domain of Human Phosphodiesterase 4B2B in Complex with A Quinoline Inhibitor, PDB code: 3gwt:
Jump to Arsenic binding site number: 1; 2; 3; 4; 5;
In total 5 binding sites of Arsenic where determined in the Catalytic Domain of Human Phosphodiesterase 4B2B in Complex with A Quinoline Inhibitor, PDB code: 3gwt:
Jump to Arsenic binding site number: 1; 2; 3; 4; 5;
Arsenic binding site 1 out of 5 in 3gwt
Go back to
Arsenic binding site 1 out
of 5 in the Catalytic Domain of Human Phosphodiesterase 4B2B in Complex with A Quinoline Inhibitor
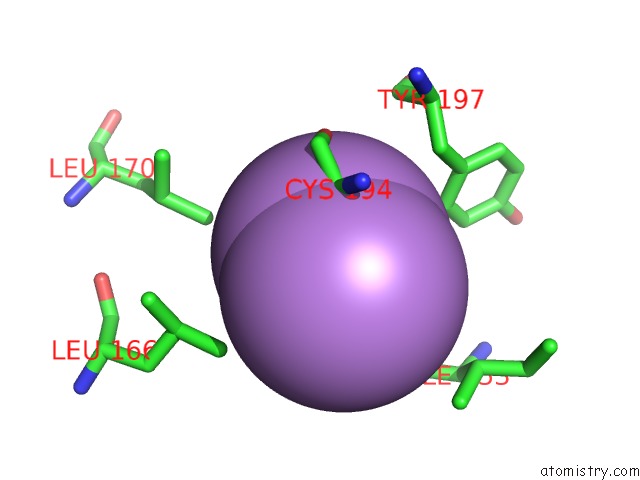
Mono view
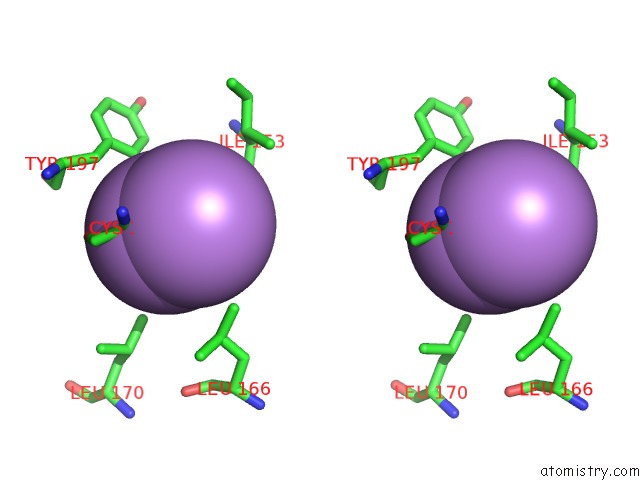
Stereo pair view

Mono view

Stereo pair view
A full contact list of Arsenic with other atoms in the As binding
site number 1 of Catalytic Domain of Human Phosphodiesterase 4B2B in Complex with A Quinoline Inhibitor within 5.0Å range:
|
Arsenic binding site 2 out of 5 in 3gwt
Go back to
Arsenic binding site 2 out
of 5 in the Catalytic Domain of Human Phosphodiesterase 4B2B in Complex with A Quinoline Inhibitor
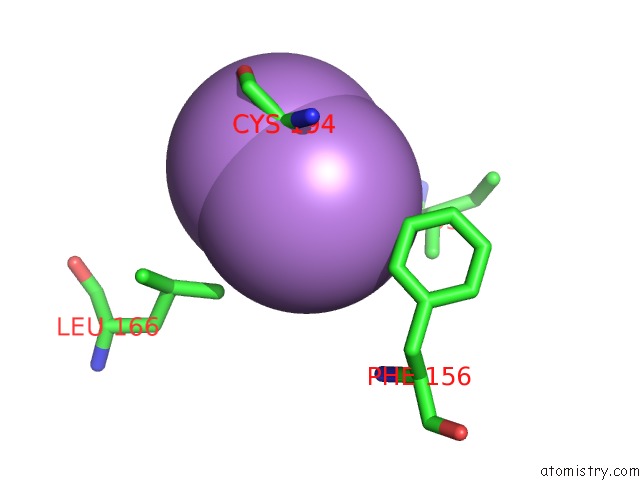
Mono view
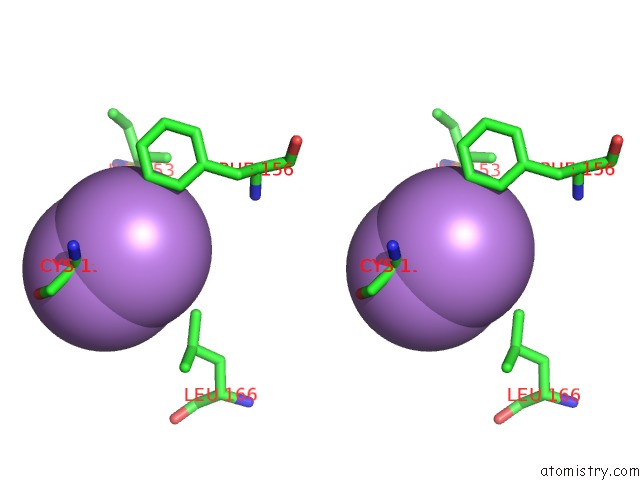
Stereo pair view

Mono view

Stereo pair view
A full contact list of Arsenic with other atoms in the As binding
site number 2 of Catalytic Domain of Human Phosphodiesterase 4B2B in Complex with A Quinoline Inhibitor within 5.0Å range:
|
Arsenic binding site 3 out of 5 in 3gwt
Go back to
Arsenic binding site 3 out
of 5 in the Catalytic Domain of Human Phosphodiesterase 4B2B in Complex with A Quinoline Inhibitor
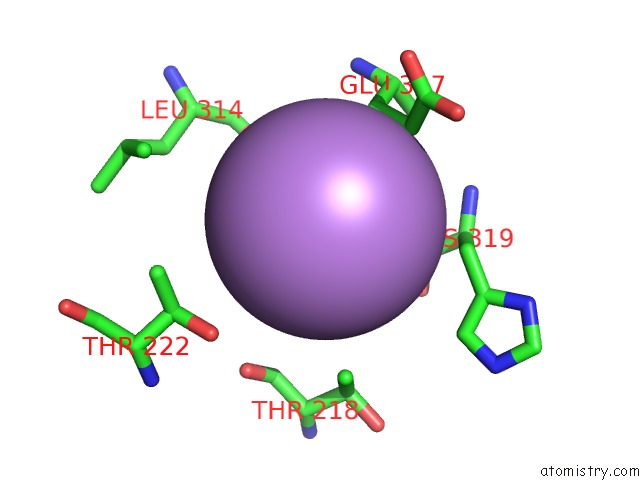
Mono view
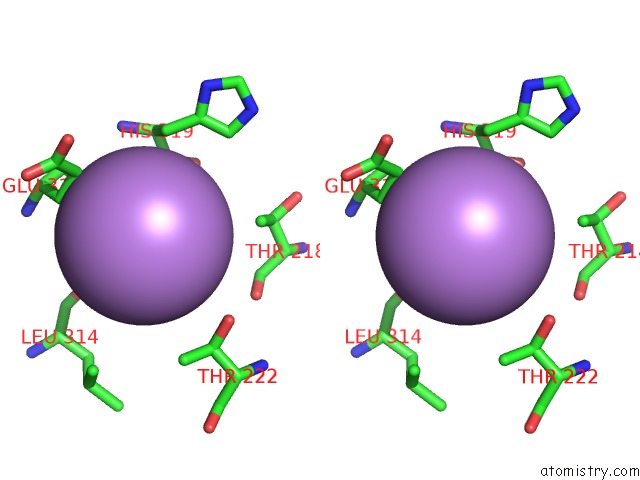
Stereo pair view

Mono view

Stereo pair view
A full contact list of Arsenic with other atoms in the As binding
site number 3 of Catalytic Domain of Human Phosphodiesterase 4B2B in Complex with A Quinoline Inhibitor within 5.0Å range:
|
Arsenic binding site 4 out of 5 in 3gwt
Go back to
Arsenic binding site 4 out
of 5 in the Catalytic Domain of Human Phosphodiesterase 4B2B in Complex with A Quinoline Inhibitor
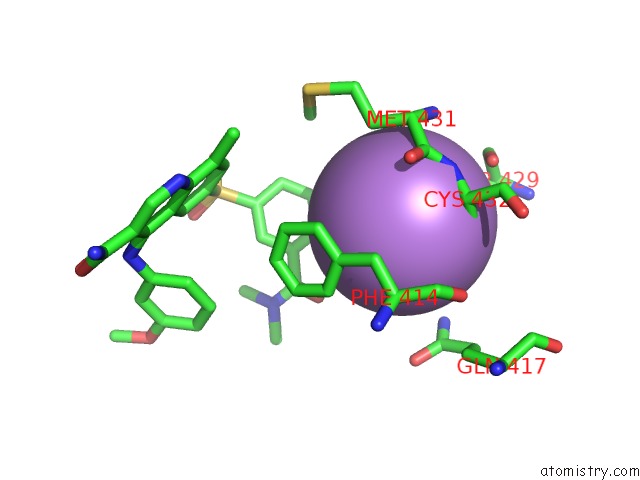
Mono view
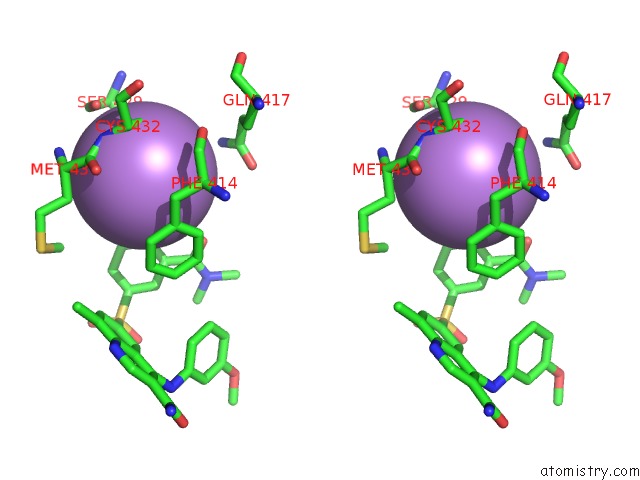
Stereo pair view

Mono view

Stereo pair view
A full contact list of Arsenic with other atoms in the As binding
site number 4 of Catalytic Domain of Human Phosphodiesterase 4B2B in Complex with A Quinoline Inhibitor within 5.0Å range:
|
Arsenic binding site 5 out of 5 in 3gwt
Go back to
Arsenic binding site 5 out
of 5 in the Catalytic Domain of Human Phosphodiesterase 4B2B in Complex with A Quinoline Inhibitor
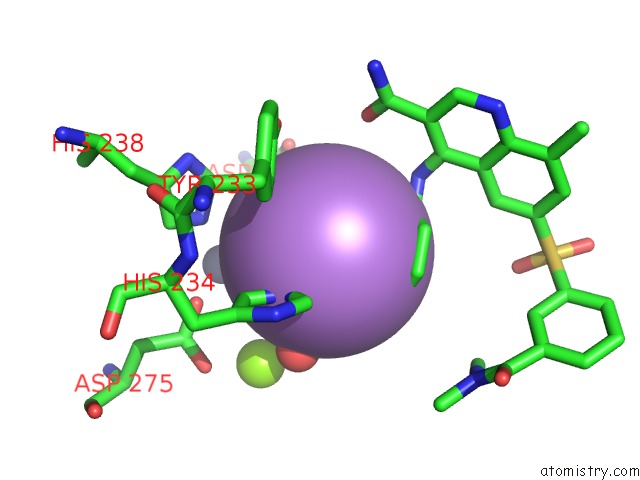
Mono view
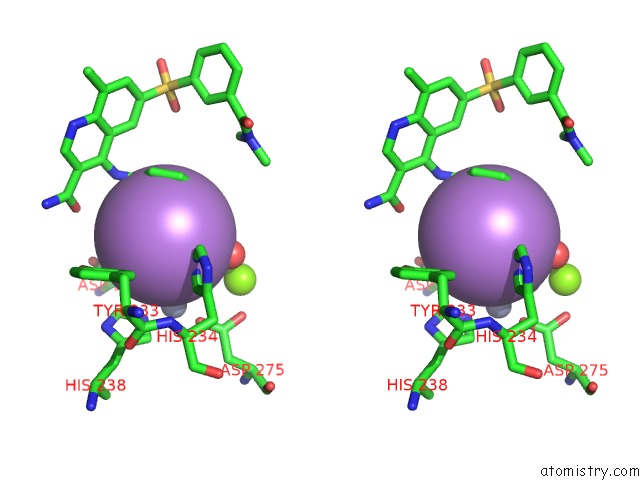
Stereo pair view

Mono view

Stereo pair view
A full contact list of Arsenic with other atoms in the As binding
site number 5 of Catalytic Domain of Human Phosphodiesterase 4B2B in Complex with A Quinoline Inhibitor within 5.0Å range:
|
Reference:
M.D.Woodrow,
S.P.Ballantine,
M.D.Barker,
B.J.Clarke,
J.Dawson,
T.W.Dean,
C.J.Delves,
B.Evans,
S.L.Gough,
S.B.Guntrip,
S.Holman,
D.S.Holmes,
M.Kranz,
M.K.Lindvaal,
F.S.Lucas,
M.Neu,
L.E.Ranshaw,
Y.E.Solanke,
D.O.Somers,
P.Ward,
J.O.Wiseman.
Quinolines As A Novel Structural Class of Potent and Selective PDE4 Inhibitors. Optimisation For Inhaled Administration. Bioorg.Med.Chem.Lett. V. 19 5261 2009.
ISSN: ISSN 0960-894X
PubMed: 19656678
DOI: 10.1016/J.BMCL.2009.04.012
Page generated: Sun Jul 6 23:23:54 2025
ISSN: ISSN 0960-894X
PubMed: 19656678
DOI: 10.1016/J.BMCL.2009.04.012
Last articles
K in 2A1OK in 2A1N
K in 2A1M
K in 2A0Q
K in 1ZZ3
K in 1ZZ0
K in 1ZZ1
K in 2A0L
K in 1ZZN
K in 1ZWI